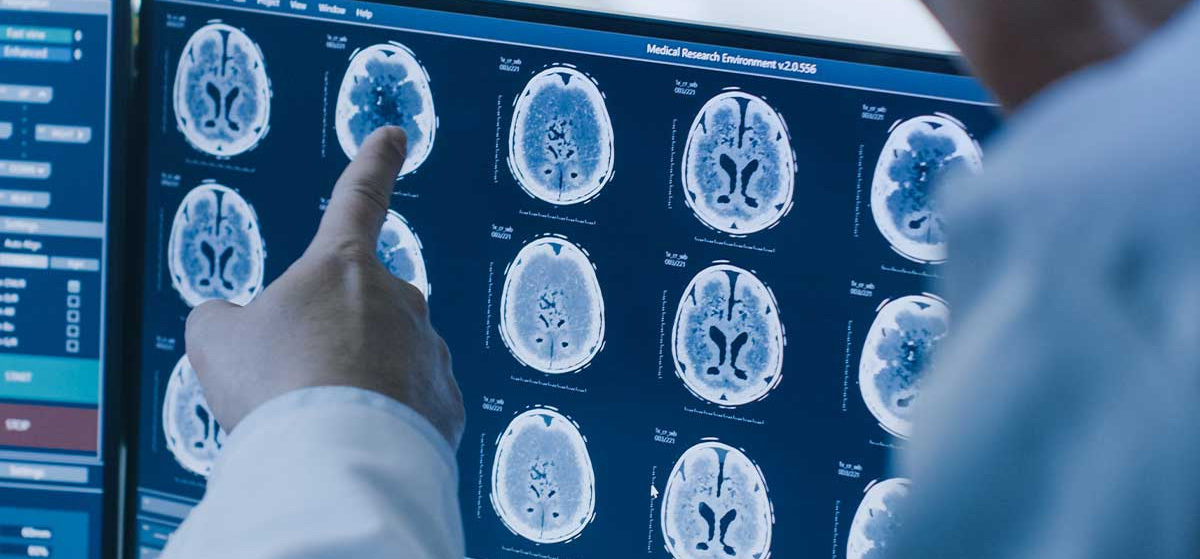
Efficacy Criteria Consulting
When IRC (Independent Review Committee) serves as the primary study endpoint in key registration clinical trials, selecting the right efficacy assessment criteria enables objective and authentic presentation of clinical trial data, strongly supporting sponsors’ new drug applications. we provide specialized consulting services on selection of evaluation criteria for different indications during the clinical trial protocol design phase;